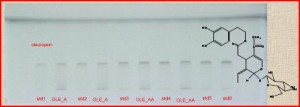
In the present study, HPTLC methods were developed, validated and compared to analytical techniques routinely used in the laboratory. High Performance Thin Layer Chromatography (HPTLC) is a simple, modern analytical densitometric analysis technique [1-5]. It is the optimum combination of modern equipment, solid theoretical foundation and standardized methodology.
2.4 Kg of aerial parts of Genista halacsyi were extracted with methanol affording 20 gr of dry extract (Ghal). HPTLC: A semi-automated sample applicator, CAMAG Linomat 5, was used for spotting HPTLC plates and a densitometric UV scanner, CAMAG Scanner type 3, was used for plate analysis. Both instruments were controlled by Wincats software version 1.4.4. TLC plates 20 × 10 cm, silica gel 60 coated on aluminium foil were used. Samples were applied as 6 mm bands using the applicator. Plates were developed to a height of 8 cm in a glass chamber saturated for 15–20 min with H2O/ACN 70/30 + 1% Acetic Acid and scanned at various wavelengths using the UV scanner. Quantitative evaluation was done via peak areas. Spot identification was based on Rf value comparison with standards, which was confirmed by a UV scan from 200 to 400 nm. HPLC was performed with a Thermo Finnigan HPLC system (ThermoFinnigan, San Jose, CA) connected to a Spectral System UV2000 PDA detector and an autosampler. ChromQuest 2.1 software was used for the management of the data. Column was Supelco RP-18 HS C18, 250 x 4.6 mm i.d., 5.0 μm (Discovery). The mobile phase was H2O + 1% acetic acid/MeOH in gradient method. Elution was monitored at 240 nm and the injection volume was 20 μl. Nuclear magnetic resonance (NMR) spectra were obtained on Bruker 600 MHz spectrometers using MeOD (Aldrich) as solvent. Coumaric acid was used as an internal standard. 1.6 gr of olive leaves were extracted with acetone affording 388 gr of dry extract (OLE_A). 200 mg of OLE_A were enriched with the active compounds by precipitation with CH2Cl2:MeOH 88:2 (OLE_AA). Pure oleuropein, isolated in our laboratory by means of FCPC was used as a standard. Stock solution (1 mg/ml) in MeOH was used for the standard calibration curves.
1. Quantification of oleuropein, an important biophenol in differently processed extracts of olive leaves.
Calibration curves were constructed for HPLC, HPTLC, NMR by plotting peak area against concentration over the range 25-150 μg/ml, 1.5-4 μg and 1-10 mg, respectively. For the quantitification of oleuropein with HPTLC 5.5 μl from stock solution (1 mg/ml) in MeOH of OLE_A and 5.0 μl from OLE_AA correspondingly. 150 μg/ml from each extract were injected to HPLC (made from the same stock solutions) and 8 mg (taken from the same stock solutions) were measured at NMR, for comparison of the oleuropein content. All the analysis were performed in triplicate.
The results from the analysis indicate that HPTLC methods are highly reproducible, reliable and most importantly in good agreement with routinely used methods (HPLC, NMR). HPTLC can be used to standardize the active constituents of herbal extracts.
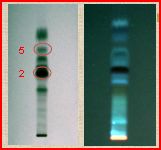
2. Fingerprinting of the initial crude methanolic extract of an endemic Greek species, namely Genista halacsyi.
4 μl of stock solution (10 mg/ml) in MeOH of Ghal were applied on the TLC plate and was observed at 254 nm and 366 nm. Mainly isoflavonoids, O-glycosides and 8-C-glycosides, were isolated. HPTLC fingerprinting profile can be used as a diagnostic tool to determine the quality and purity of a plant material and to identify marker compounds.
3. Possibility of using HPTLC in counter-current chromatography (CPC) for determination of partition coefficients (KD).
HPTLC estimated Rf were used for the determination of KD for elaboration at CPC analysis. The above mentioned methanolic extract of G. halacsy was used and the Rf of compounds 2 and 5 were calculated. Gradient solvent solutions (6 systems) of hexane:ethyl acetate:butanol:methanol:water were developed, keeping methanol and water amounts stable in all systems. The order of elution was compared with HPLC analysis, which is typically used for KD determination. HPTLC can efficiently be used in methodology creation in CPC, being as accurate as HPLC and in parallel more cost effective, faster and simplified.
1. Vanhaelen-Fastre, R.J. et al. (2000) J. Chromatogr. A 868:269-276
2. Yadav, D. et al. (2011) J. Sep. Sci. 34:286-291
3. Rashmi, et al. (2011) Phcog J. 3:41-44
4. Dhalwal, K. et al (2010) J. Med. Plants Res. 4:1289-1296
5. Plocharz, P. et al (2010) J. Chromatogr. A 1217:4868-4872