The aim of the project is to discover and carry to the stage of development candidates, plant derived small molecules with potential as new cosmetic agents. Greece, in particular, is well known for the richness and diversity of its flora that consists of at least 6300 species and subspecies. A significant proportion of Greek land is covered with high mountains, which form some of the most interesting botanical localities. Over 15% of Greek flora is endemic and it is not found anywhere else in the world. Despite their great importance only a small proportion, approximately 10%, of the classified plants have been investigated and chemically characterized. They produce a wide variety of the so-called “secondary metabolites” or “small molecules”, which are usually candidates for drugs, cosmetics or other technological developments, directly or as an inspiration for unnatural products.
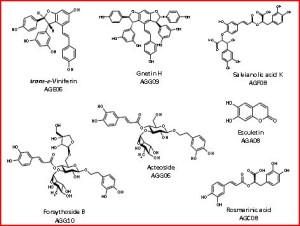
The selected compounds belong to several categories: Flavonoids, alkaloids, terpenes, lactones, lignans, coumarins, quinones, acetophenones, stilbenes, thiophenes, polyacetylenes, phenolic acids, phenylethanoid glycosides and benzofurans. From an existing collection comprising a total of > 1000 compounds, a hundred of pure small molecules were selected for the evaluation of their antioxidant and anti-tyrosinase properties.
The free radical formation induced by UV is at the origin of most cellular and molecular processes leading to premature skin aging. Protection can be obtained via UV-blocking agents or antioxidants and radical scavengers. Radical scavenging activity of selected small molecules against stable DPPH. (2,2-diphenyl-2-picrylhydrazyl hydrate) was determined spectrophotome-trically. When DPPH. reacts with an antioxidant compound, which can donate hydrogen, the amount of free radical is reduced. The changes in colour (from deep—violet to light—yellow) were measured at 515 nm on a plate reader (TECAN). The decrease in optical density of DPPH induced by test samples in relation to the control was used to calculate the antioxidant activity. IC50 values, which correspond to the required amount of each sample to scavenge 50% of the DPPH free radicals, were calculated for the most active compounds (% Inh > 80 at 75 μg/ml).
Anti-tyrosinase (anti-hyperpigmentation) activity of the selected molecules was determined using the 96-well microplate method described by Masuda et al. [2], based on their capacity to inhibit the activity of mushroom tyrosinase when co-incubated with its substrate L-tyrosine and its co-substrate L-DOPA. Initially, compounds dissolved in DMSO were tested at final concentration 150 μg/ml and the percentage inhibition of tyrosinase activity was determined. IC50 values were calculated for the most promising molecules (% Inh > 80 at 150 μg/ml).Tyrosinase is a multifunctional, glycosylated, and copper-containing oxidase, which catalyzes the first two steps in mammalian melanogenesis. Although melanin has mainly a photoprotective function, the accumulation of an abnormal amount of melanin in different specific parts of the skin resulting in more pigmented patches might become an esthetic problem.
The analysis of biological screening data proved that phenolic derivatives, phenylpropanoid glycosides and stilbenes are the most promising secondary metabolites. However, other scaffolds also showed good antioxidant or/and anti-tyrosinase activity. Based on this analysis, small molecules databases and chemotaxonomy literature were mined for distribution of the desirable scaffolds, and for decoration patterns around these scaffolds. The Greek flora was analyzed for species/genera/families expected to contain the desired scaffolds and decoration patterns and a plant collection of around 450 species, belonging to 68 plant families of the Greek flora, is established.
A state-of-the-art technology platform for miniaturized natural product discovery will generate focused sub-libraries around these privileged scaffolds. Further evaluation of these sub-libraries will lead to the development of novel products in cosmetics with new or improved properties over existing active ingredients.
[1] Polunin, 0. (1980) Flowers of Greece and the Balkans. Oxford University Press, Oxford
[2] Masuda T. et al. Biosci. Biotechnol. Biochem., 2007, 71(9), 2316-2320